Customized precision for the highest pharmaceutical standards
When structural limitations leave no room for maneuver and the highest GMP requirements must be met, standard solutions are not an option. This was precisely the situation Chanelle faced: Five drum lifters had to be integrated into tightly dimensioned clean rooms—under conditions that required millimeter-precise planning and maximum flexibility.
Challenges
Chanelle had to install five stationary drum lifters in several compression rooms in a pharmaceutical cleanroom area—in rooms whose structural conditions allowed little leeway. There were only a few centimeters between the barrel, tablet press, and ceiling, which made it impossible to use standard technology. Each of the five lifting columns had different swivel angles, height requirements, and space restrictions. Precise layouts were hardly available, so essential dimensions had to be planned as “best guesses” and designed to be adjustable on site. In general, the following requirements also applied:
- Cleanroom suitability, GMP-compliance and safe operation
- Handling of 200-L stainless steel drums
- 0–180° inversion, precise (on-site) adjustable height stops, ergonomic operation
- Documentation, IQ/OQ capability, and full validatability
Solution
In order to meet the demanding structural, technical, and regulatory requirements, a special solution ("tailor-made") was developed for each individual lifting column:
1. Specially adapted offset arm
The support arm has been completely redesigned to balance the critical heights – pick-up height, dump height, maximum lift – precisely within the available space. A standard arm would not have been able to achieve this combination.
2. Highly flexible adjustment options
All critical parameters (e.g., swivel angle, height stops, positioning) were designed to be mechanically adjustable. This allowed an experienced technician on site to adjust each column precisely.
3. Cleanroom and GMP-compliant pharmaceutical design
Smooth stainless steel surfaces, no sharp edges, easy cleaning and maintenance, suitable for use with 200 L stainless steel drums, manual, secure clamping, validatable documentation (GA drawings, IQ/OQ documents, MOC certificates, etc.)
4. Complete functional integration
All functions—hydraulic lifting, 0–180° inversion, motorized rotation, hold-to-run operation, height stop, overload protection—have been integrated and optimized for cleanroom production.
5. Timely implementation
Despite the tight time frame and the extended coordination phase, delivery was even able to take place slightly earlier than planned.
Benefit for the customer
1. No structural alterations necessary
Thanks to the precise customization, Chanelle was able to integrate the lifting columns without any modifications. According to the project manager at Chanelle, the alternative would have been to raise the false ceiling, including ventilation, lighting, and installations—an enormous undertaking that was completely avoided.
2. Safe, ergonomic, and GMP-compliant working
The solution meets all pharmaceutical requirements for cleanroom suitability, OEB-friendly handling, and operator safety. → Less physical strain and significantly lower risk of contamination.
3. Reproducible and precise process flows
Thanks to adjustable height stops, secure barrel clamping, and controlled inversion, Chanelle can standardize powder feeding processes and repeat them in a validated manner.
4. Future-proof and maximum flexibility
The flexibly adjustable design allows for adjustments if room layouts or production requirements change.
5. Complete validity
All necessary documents (DQ/FS/FDS, IQ/OQ, certificates, technical documentation) were provided—a must for pharmaceutical GMP environments.
Result
The project was successfully implemented on time, with the required quality and despite its high complexity – a real “sniper operation,” as described internally: at the right time, accurate to the millimeter and despite sudden additional challenges. Chanelle now has a complete, valid and reliable drum lifter solution that:
- Ensures ergonomic and safe operation
- Complies with OEB and GMP standards
- Improves efficiency in powder feeding
- Integrates perfectly into the spatial conditions
- Could be implemented without structural modifications
A sustainable investment in safety, process quality, and future viability.

The barrel is transported safely and ergonomically to the system using a barrel trolley and is easily inserted into the lifting column. 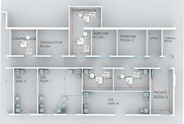
existing room layout 
Model in the quotation phase for visualization for the customer 
during commissioning 
docked barrel on the tablet press 
maximum utilization of ceiling height





