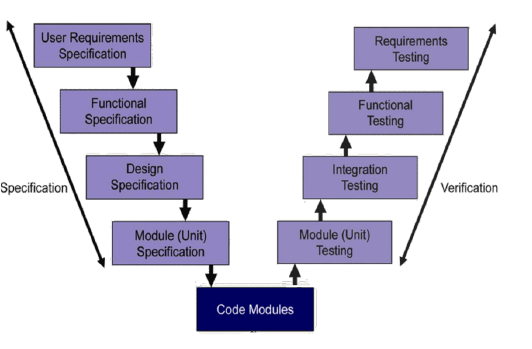
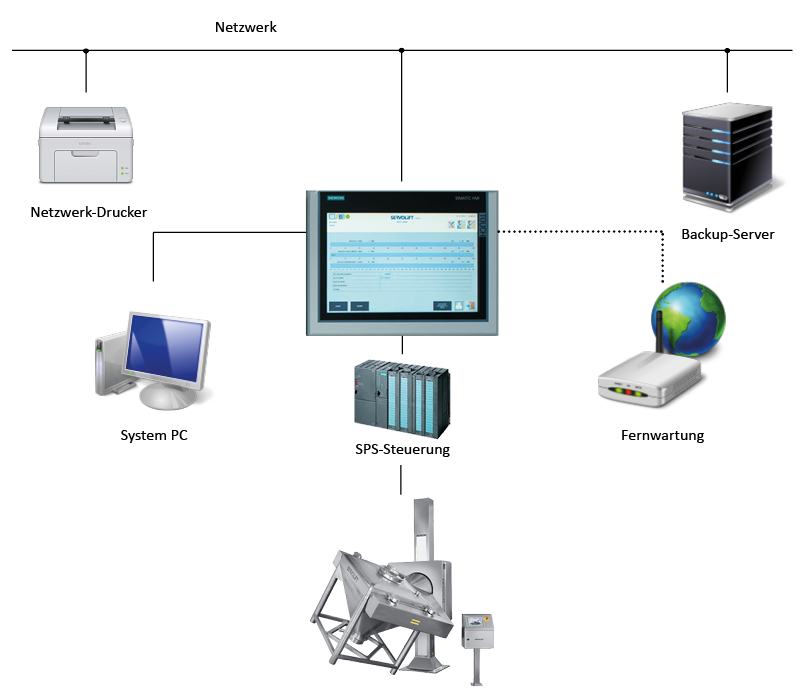
- Implementing “Electronic Records/Electronic Signatures” in observance of FDA Guideline 21 CFR Part 11
- Windows-based security implementation with clear assignment of user recognition and passwords, password lifecycles, password history and defined password lengths
- Complete audit trail system with encrypted, check sum-secured display of all significant user actions, alarm messages and process data
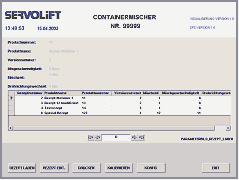
- Formula management
- Creation of batch protocols
- Presentation of all operating statuses and error messages from the test machines
- Ability to calibrate the system
- Regulated remote maintenance capability
- Creation of qualification documents and implementation of tests for installation qualification (IQ) and functional qualification (OQ)